Science Spotlight
Elucidating an Improved Live-Imaging Method for Adult Fish: A New Age of Zebrafish Englightenment
By Timothy M. Hufford
One of the strengths intrinsic to zebrafish as a model for developmental and cell biology is optical transparency of embryos, making them amenable to various live-imaging methods. While live-imaging of embryos has been and will undoubtedly be a major tool to study aspects of early development, high-resolution images of live adult zebrafish has been a challenge. To make advances in translational research using zebrafish, there is an acute need for robust live-imaging methods of adult fish.
Imaging of adult zebrafish requires careful dosing of anesthetic to avoid thrashing and movements that interrupt imaging of cells/tissues of interest. Previous methods have used intubation to providing rhythmic flow of water and anesthetic (Tricaine, or MS-222) through a peristaltic pump (Xu et al., 2015). A limitation in this approach is that fish must be embedded in agarose to reduce movement from the pump, requiring careful removal of agarose from the cell/tissue area to be imaged. Any residual agarose that is not removed will dramatically reduce clarity and signal intensity of fluorescent reporters (Xu et al., 2015). Embedding adult fish in agarose can also run the risk of covering the gills, potentially causing death of the animal (Castranova et al., 2021). Another area of concern is maintenance of water levels in the imaging chamber: adult fish must be surrounded by water, which must be steadily replenished to keep the fish healthy for long-term imaging or repeated imaging. Thus, preventing overflow is a critical consideration (Castranova et al., 2021; Cox et al., 2018). Previous approaches relied on manual replenishment of water every few hours or implementation of a water sensor to shut off inflow when outflow is blocked (Cox et al., 2018; Xu et al., 2015). These methods allow long-term imaging, but still require regular monitoring.
To overcome these challenges in imaging adult fish, Castranova and others have developed an innovative and easily reproducible imaging “rig” (Castranova et al., 2022)(see figure, schematic on right). The rig expands upon the previous methods, utilizing two outflow tubes and an emergency overflow tube, as well as the use of a pulse dampener to the peristaltic pump. The pulse dampener – the part that reduces vibrational “noise” from the peristaltic pump – was made with a plastic Erlenmeyer flask with tube fittings that were glued using epoxy. This home-made pump offers both ease of cleaning and affordability compared to commercially available dampeners. The method is also very accessible and customizable, with a CAD file for 3D-printing made available for in-house design of the imaging setup. The authors demonstrated the robustness of their rig by imaging the process of neutrophil recruitment in wound healing. The fish reporter line used to test the rig were double transgenic casper zebrafish, which mark lymphatic vessels and neutrophils (see figure), as well as Mexican Cavefish (Astyanax mexicanus). The authors demonstrate that prolonged, overnight imaging (at least 20 hours) and repeated intubation of the same fish (4 times, 3.5 hours each) from this setup does not appreciably interfere with the processes under study.
The development of this imaging rig addresses previous challenges of imaging adult fish and provides an attractive approach for long-term imaging that can allow the same fish to be intubated and imaged multiple times, including overnight imaging. Dan Castranova reflected on the progression of the development of imaging technology in zebrafish. “When I started working with zebrafish, the vast majority of work was done in 24 hours [post-fertilization]. Now, 6-7 days is more common.” However, some critical aspects of development do not even begin within this window of time. “Development of intracranial lymphatics occurs after 2 weeks”, Dan remarked. The intubation chamber was used in a previous publication by Dan and Brant Weinstein (Castranova et al., 2021). Following this, Brant encouraged Dan to further develop and test the intubation chamber, which could become a standard approach. “We aren’t the first to intubate adult fish, but the previous approaches were designed for the logistics of an upright microscope. Inverted scopes are becoming increasingly common, and our chamber was developed and tested for use on these.”
Dan spoke on the learning curve of intubation, “one of the biggest challenges is getting the tube into the fish’s mouth. It’s almost like learning how to microinject”, a very relatable analogy for many zebrafish researchers. Dan also clarified some key points on the chamber and their objective lenses. The 20X objective used in this work is a long working distance air objective, and so it did not require immersion oil. Silicone grease is used to attach the cover glass to the intubation chamber, and it would be problematic for this to contact oil required by immersion objective lenses. Thus, the intubation chamber may require custom assembly to suit the needs of each researcher depending on the project and the imaging equipment available. Using this method, zebrafish researchers can now easily image adult fish to better observe cellular processes in real-time, e.g., artery formation during fin regeneration, intracranial lymphatics, cancer cell metastasis in xenografted fish, and adult brain imaging (Benjamin & Hynes, 2017; Castranova et al., 2021; Xu et al., 2014; Xu et al., 2015).
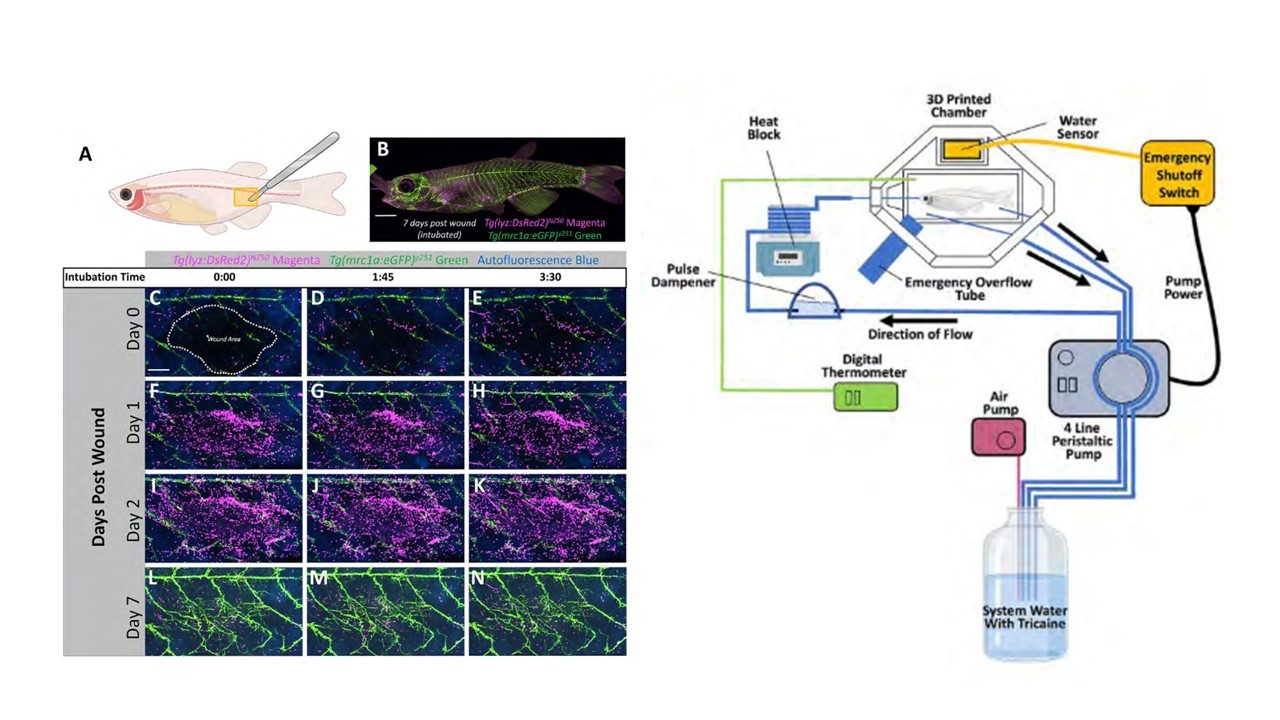
Figure adapted from Castranova et al. 2022. Left: Casper zebrafish can be imaged/intubated repeatedly to study wound healing over the course of seven days. Lymphatic vessels in green, neutrophils in magenta. Right: Schematic detailing the imaging rig.
Benjamin, D. C., & Hynes, R. O. (2017). Intravital imaging of metastasis in adult Zebrafish. BMC Cancer, 17(1), 660. https://doi.org/10.1186/s12885-017-3647-0
Castranova, D., Samasa, B., Galanternik, M. V., Jung, H. M., Pham, V. N., & Weinstein, B. M. (2021). Live Imaging of Intracranial Lymphatics in the Zebrafish. Circulation research, 128(1), 42-58. https://doi.org/doi:10.1161/CIRCRESAHA.120.317372
Castranova, D., Samasa, B., Venero Galanternik, M., Gore, A. V., Goldstein, A. E., Park, J. S., & Weinstein, B. M. (2022). Long-term imaging of living adult zebrafish. Development, 149(4). https://doi.org/10.1242/dev.199667
Cox, B. D., De Simone, A., Tornini, V. A., Singh, S. P., Di Talia, S., & Poss, K. D. (2018). In Toto Imaging of Dynamic Osteoblast Behaviors in Regenerating Skeletal Bone. Curr Biol, 28(24), 3937-3947.e3934. https://doi.org/10.1016/j.cub.2018.10.052
Xu, C., Hasan, S. S., Schmidt, I., Rocha, S. F., Pitulescu, M. E., Bussmann, J., Meyen, D., Raz, E., Adams, R. H., & Siekmann, A. F. (2014). Arteries are formed by vein-derived endothelial tip cells. Nature Communications, 5(1), 5758. https://doi.org/10.1038/ncomms6758
Xu, C., Volkery, S., & Siekmann, A. F. (2015). Intubation-based anesthesia for long-term time-lapse imaging of adult zebrafish. Nature Protocols, 10(12), 2064-2073. https://doi.org/10.1038/nprot.2015.130
About the Authors
 Tim Hufford is a PhD candidate in the lab of Dr. Rachel Brewster at the University of Maryland, Baltimore County, where he studies the mechanisms that help zebrafish trigger a protective & metabolically suppressed state in anoxia, or the absence of oxygen.
Tim Hufford is a PhD candidate in the lab of Dr. Rachel Brewster at the University of Maryland, Baltimore County, where he studies the mechanisms that help zebrafish trigger a protective & metabolically suppressed state in anoxia, or the absence of oxygen.
 Dan Castranova is the Aquatic Research Specialist in the lab of Dr. Brant Weinstein at the National Institute of Child Health and Human Development (NIH/NICHD) in Bethesda, Maryland. There, Dan found a passion for imaging zebrafish (and cavefish) to shed light on vascular development of the circulatory and lymphatic systems.
Dan Castranova is the Aquatic Research Specialist in the lab of Dr. Brant Weinstein at the National Institute of Child Health and Human Development (NIH/NICHD) in Bethesda, Maryland. There, Dan found a passion for imaging zebrafish (and cavefish) to shed light on vascular development of the circulatory and lymphatic systems.
 Dr. Brant Weinstein is a Senior Investigator and the Associate Scientific Director of the Division of Developmental Biology at the National Institute of Child Health and Human Development (NIH/NICHD) in Bethesda, Maryland. His lab is one at the forefront of research on vascular development, having contributed key mechanistic insights to understanding the formation of the circulatory and lymphatic systems, and having developed many useful transgenic reporters and imaging methods to better study these processes.
Dr. Brant Weinstein is a Senior Investigator and the Associate Scientific Director of the Division of Developmental Biology at the National Institute of Child Health and Human Development (NIH/NICHD) in Bethesda, Maryland. His lab is one at the forefront of research on vascular development, having contributed key mechanistic insights to understanding the formation of the circulatory and lymphatic systems, and having developed many useful transgenic reporters and imaging methods to better study these processes.
Would you like to have a mentored science writing experience as a Science Spotlight author for NewsSplash? If you are interested, please contact Cecilia Moens (cmoens@fredhutch.org) or Karuna Sampath (K.Sampath@warwick.ac.uk) for more details about joining our team.
How Zebrafish Eyes Stretch to Get Into Shape
By Daphne Daniel
Guest Editor Kara Cerveny
Though vertebrate embryos are incredibly diverse with regard to size, shape, and speed of development, they all employ localized changes in cell shape to generate specialized tissues and organs. One problem that embryos encounter is how to efficiently turn a flat sheet of cells into a three-dimensional ball. This challenge is illustrated (and solved) during optic cup morphogenesis when a flattened single-layered optic vesicle turns into a rounded bilayered optic cup. The layers of the optic cup begin as undifferentiated neural progenitor cells and differentiate into the neural retina (NR, inner layer) and retinal pigment epithelium (RPE, outer layer) as development proceeds. A collection of studies has identified chemical signals such as growth factors and physical signals such as cell-cell and cell-ECM connections as essential for the coordinated formation and morphogenesis of the layers of the developing eye (reviewed in Cardozo et al., 2020 and Casey et al., 2021). Although changes in the RPE have been observed during optic cup morphogenesis, it has been unclear whether and how the RPE itself guides optic cup morphogenesis. To address this question, Moreno-Mármol et. al. (2021) performed a series of imaging experiments in zebrafish embryos using genetic and pharmacological tools.
The authors first generated a new transgenic line that specifically labels RPE cells beginning at the earliest stages of optic cup morphogenesis. Using this new tool to capture time-resolved images of cell shape changes the NR and RPE, the authors found that RPE cells began as columnar, transitioned to cuboidal, and finally achieved their final flattened squamous shape. The effect of these cell-shape changes was an overall increase in RPE surface area, but a decrease in RPE thickness.
To investigate how changes in RPE cell shape influence optic cup morphogenesis, the authors disrupted the actomyosin cytoskeleton using a azidoblebbistatin (ableb), a photoactivatable derivative of the myosin II inhibitor, blebbistatin. Previous work has shown that whole-body application of blebbistatin disrupts optic vesicle folding (Nicolás-Pérez et. al, 2016). To disentangle the effects of myosin II activity in the NR versus RPE, the authors locally inhibited myosin II activity in either the NR or RPE by first soaking embryos in ableb and then using two-photon microscopy to activate ableb at specific times and in precise locations. Ableb-treated embryos with irradiated RPEs showed similar results to blebbistatin-treated embryos, with a less folded, flattened optic cup and cuboidal RPE cells. Ableb-treated embryos with irradiated NRs showed a slightly less folded optic cup and RPE cells that appeared identical to control embryos. Together these data suggest that RPE cell flattening occurs independently of the NR and that myosin II mediated cell shape changes in the RPE alone contribute to optic cup morphogenesis.
To examine the evolutionary underpinnings of optic cup morphogenesis, the authors compared RPE development in zebrafish with more slowly developing amniotes: chicks, mice, and humans. In contrast to zebrafish, the amniotes exhibited increased cell proliferation in the RPE, allowing the RPE to increase in surface area without decreasing in thickness. In zebrafish, the change in RPE surface area was accompanied by a marked decrease in RPE cell proliferation. One possible explanation for this difference is that for rapidly developing species like zebrafish it is more beneficial to undergo rapid cell shape changes than to conduct resource-intensive rounds of cell division that also take upwards of 4-6 hours to complete. In amniote species that spend more time generating their eyes, the time taken to expand the RPE through cell proliferation may be more robust to perturbations.
Together, all of the data from Moreno-Mármol’s study set a firm foundation for further investigations into the various routes to OC development that natural selection has favored. There are still, however, many open questions including how changes in the RPE influence changes in the NR and the cellular and molecular connections between these two tissues drive optic cup morphogenesis. Other open questions include the nature of the trade-offs, either physiological or evolutionary, associated with having a non-proliferating RPE and how these differing approaches to RPE development - proliferating or flattening of the cell - emerged from the vertebrate common ancestor.
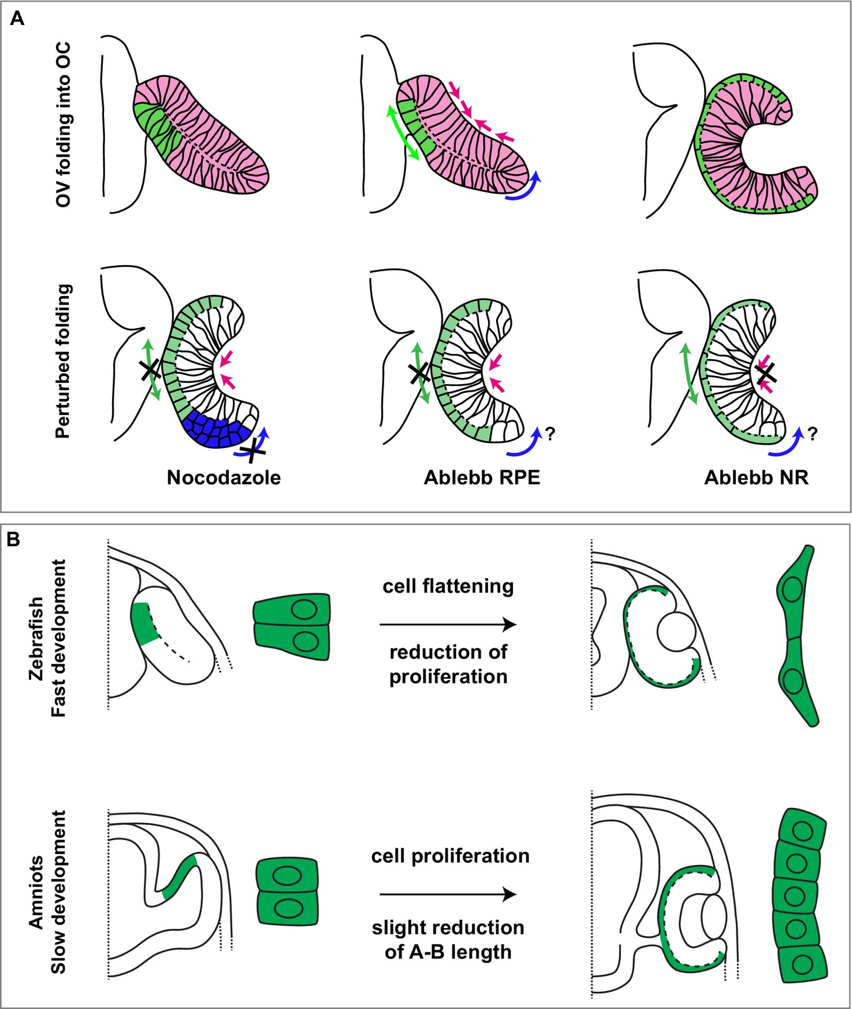
Summary image, Figure 8 reproduced from Moreno-Mármol, 2021.
About the Authors
Tania Moreno-Mármol is a science professor presently teaching at the Santísmo Sacramento College in Madrid, Spain. She received her PhD in Molecular Biosciences in 2019 from the CBMSO and conducted research for this publication during her time at the CBMSO and at the University Autónoma of Madrid. Paola Bovolenta is currently a research professor and the head of the development and differentiation department at the CBMSO in Madrid, Spain. Paola received her PhD from the New York University, School of Medicine in 1986 and is interested in developmental neurobiology and its potential impact on human health. She is presently a member of the European Molecular Biology Association (EMBO) and served as a supervisor and corresponding author for this study.
Daphne Daniel wrote this piece as part of an assignment for a seminar titled, Special Topics in Developmental Neurobiology (BIOL 431) in the spring of 2022. Daphne is a rising senior majoring in Biology at Reed College in Portland, OR. Starting in the fall, she plans to study retinoic acid responsive genes in the developing zebrafish retina for her senior thesis in the Cerveny Lab and apply for graduate study in a to-be-determined field, but hopefully something related to fish and the ocean. She enjoys science communication, illustration, and watching nature shows (her current favorite is Prehistoric Planet).
References
- Moreno-Mármol T, Ledesma-Terrón M, Tabanera N, Martin-Bermejo MJ, Cardozo MJ, Cavodeassi F, Bovolenta P (2021). Stretching of the retinal pigment epithelium contributes to zebrafish optic cup morphogenesis eLife 10:e63396
- Eiraku M, Takata N, Ishibashi H, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
- Casey MA, Lusk S, Kwan KM (2021). Build me up optic cup: Intrinsic and extrinsic mechanisms of vertebrate eye morphogenesis. Dev Biol 476, 128-136
- Nicolás-Pérez M, Kuchling F, Letelier J, Polvillo R, Wittbrodt J, Martínez-Morales JR (2016) Analysis of cellular behavior and cytoskeletal dynamics reveal a constriction mechanism driving optic cup morphogenesis eLife 5:e15797
Would you like to have a mentored science writing experience as a Science Spotlight author for NewsSplash? If you are interested, please contact Cecilia Moens (cmoens@fredhutch.org) or Karuna Sampath (K.Sampath@warwick.ac.uk) for more details about joining our team.




