In Memoriam: Friedrich Bonhoeffer (1932-2021)
With the death of Friedrich Bonhoeffer on January 29, 2021, developmental neurobiology has lost one of its founders and pre-eminent pioneers. His discoveries of the molecular mechanisms of axon guidance and map formation in the visual system have fundamentally and indelibly transformed the field.
Friedrich Bonhoeffer studied physics in Göttingen and wrote his doctoral thesis in 1958 at the age of 26. As a postdoc, he worked at the University of California at Berkeley, where he studied the physical properties of nucleic acids and nucleoprotein complexes. In 1962, he moved to Tübingen, where he stayed for the rest of his scientific career.
In the 1960s and 1970s, Bonhoeffer and colleagues demonstrated the necessity of DNA polymerase I for the replication of prokaryotic genomes. He made this discovery with the help of ingenious in vitro assays. The invention of elegant, informative assays, which reduced complex biology to a single functional readout, was to become a hallmark of Bonhoeffer's ingenuity as he moved on to new challenges in developmental biology.
In 1972, Friedrich Bonhoeffer became Director at the Max Planck Institute for Virus Research (renamed later to Max Planck Institute for Developmental Biology). Soon thereafter, he turned his attention to neuro-embryology. The Max Planck Society grants its directors practically unlimited freedom to choose their research direction. From an US American viewpoint, it is perhaps hard to believe that Friedrich Bonhoeffer never applied for grants. Nor did he ever have to defend his expertise to a committee of peers, like an NIH study section or an ERC panel. Bonhoeffer himself credited the trust-based Max Planck system with his ability to pursue a long-term vision. The switch from DNA replication to axon guidance must have happened almost overnight. When I joined his lab in 1989 as an undergraduate, lore was passed around that some day in the mid-1970s, Bonhoeffer instructed his team to discard all lab notes and materials related to their DNA work and to begin to work immediately on culturing nerve cells.
Back then, it was unknown how neurons form specific connections during development. A debate raged between those who believed in Langley's and Sperry's concept of chemo-affinity (which we would call "molecular specificity" today) and those who assumed that connections were formed initially by chance and reinforced, or eliminated, by experience, reward or another activity-dependent process. To me, as a budding scientist in the late 1980s, the second camp appeared more vocal and far better established. But even then, the evidence weighed strongly in favor of cell-surface, molecular specificity, thanks in large part to the work that Friedrich Bonhoeffer and his team had published in the preceding decade. (To this day, this debate continues to flare up occasionally, recently re-ignited by the success of machine learning through deep neural nets, which require no pre-specified architecture, despite the claim that they are "biologically inspired".) In hindsight, I find it admirable that Bonhoeffer stayed out of this fray. This was perhaps because he was confident that the evidence would eventually prove him right. In any case, in those days, we did not talk much about other people's work. At least to us, the "action" was happening in the Bonhoeffer lab in Tübingen.
Bonhoeffer recognized that a key step in the process of forming connections was axon guidance, i. e., the capacity of axons to detect the location of their targets and navigate toward them, sometimes over centimeter-long distances. He chose the retinotectal projection as a model system. Neighboring regions of the eye are connected to neighboring regions of the tectum, such that an orderly map of the visual world is re-created in the brain. The work was initially carried out on chick embryos, and a coop for free-range chickens was set up behind the Max Planck Institute to ensure a steady supply of fertilized eggs for experiments.
Similar to his earlier work on DNA replication, Bonhoeffer approached the problem of axon guidance by developing a series of creative in vitro assays. All of them had in common that developing axons, growing out of explants of the chick retina, were confronted with a choice between substrates of varying molecular composition. The substrates for axon outgrowth were initially carpets of cells, derived from different regions of the tectum (1982); then cell membranes prepared from these cells (1987); and, ultimately, purified molecules, reconstituted in artificial lipid membranes (1995). To give an example of one of the most widely used paradigms: In the now-famous stripe assay, the embryonic retina was spread out on a sticky filter and cut into thin bars. Individual bars of retinal tissue were placed on a surface that had previously been prepared in such a way that two different membrane substrates were arranged in alternating, parallel corridors. Bonhoeffer showed that when retinal ganglion cell axons were offered membranes from the 'correct' and the 'wrong' tectal target area, then they chose the 'correct' substrate. Thus, they preferred to grow on membranes derived from their in vivo target zone.
Friedrich Bonhoeffer and his team – especially his long-time technical assistant Julita Huf must be named here – proved that this preference was due to a repulsive activity in the cell membranes from the 'wrong' target area. This realization came at a time when axon guidance (like the migration of cells) was largely attributed to differential adhesion. Bonhoeffer's discovery of a target-specific, repulsive mechanism permanently liberated the field from this dogma. Now, we know many classes of axon guidance factors, some attractive, some repulsive, some either repulsive or attractive depending on context and nature of the receptor.
With the development of this ingeniously simple assay, the door was opened to a better understanding of the molecular and cellular mechanisms underlying axon guidance. I joined Bonhoeffer's group when efforts to purify the guidance "activity" from tectal membranes were well under way. For my part, I could contribute the small insight that shallow gradients of the repellent cue were effective in halting axon growth (1992), thus recapitulating in vitro the gradient's postulated function in the tectum.
 The stripe assay work culminated in the identification of cell-surface molecules of the Ephrin-A family (and later their EphA receptors) by Uwe Drescher in Bonhoeffer's department and, in parallel, by John Flanagan and his co-workers at Harvard Medical School (1995). To many in the field, this discovery was the breakthough. Finally, we could give names and assign amino acid sequences to Sperry's long-sought chemo-affinity cues! True to character, however, Bonhoeffer considered the ephrins merely as handles to answer a much more profound question: How do gradients of molecular cues and their receptors (ephrins, Eph receptors, or whatever) steer a retinal growth cone to a unique, "individualized" position in the tectum. Alfred Gierer, Bonhoeffer's lifelong mentor and collaborator in Tübingen, had theoretically predicted a system of three or four such gradients in tectum and retina each. This problem preoccupied Bonhoeffer to the very end, and he continued to do experiments as an emeritus director, partially in collaboration with Franco Weth and Martin Bastmeyer in Tübingen and Karlsruhe. I think it is fair to say that the problem remains unsolved to this day.
The stripe assay work culminated in the identification of cell-surface molecules of the Ephrin-A family (and later their EphA receptors) by Uwe Drescher in Bonhoeffer's department and, in parallel, by John Flanagan and his co-workers at Harvard Medical School (1995). To many in the field, this discovery was the breakthough. Finally, we could give names and assign amino acid sequences to Sperry's long-sought chemo-affinity cues! True to character, however, Bonhoeffer considered the ephrins merely as handles to answer a much more profound question: How do gradients of molecular cues and their receptors (ephrins, Eph receptors, or whatever) steer a retinal growth cone to a unique, "individualized" position in the tectum. Alfred Gierer, Bonhoeffer's lifelong mentor and collaborator in Tübingen, had theoretically predicted a system of three or four such gradients in tectum and retina each. This problem preoccupied Bonhoeffer to the very end, and he continued to do experiments as an emeritus director, partially in collaboration with Franco Weth and Martin Bastmeyer in Tübingen and Karlsruhe. I think it is fair to say that the problem remains unsolved to this day.
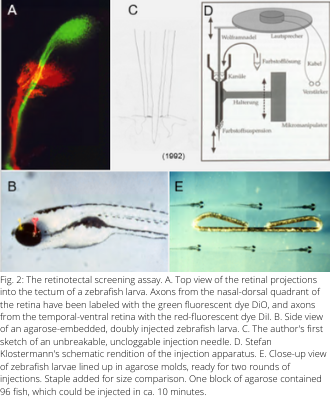 In the early 1990s, a new opportunity arose in Tübingen to decipher the molecular mechanisms of retinotectal map formation in a systematic fashion. A gigantic mutagenesis screen with zebrafish was prepared in the neighboring laboratory of his colleague Christiane 'Janni' Nüsslein-Volhard (see Figure 1). Bonhoeffer recognized the power of searching for genes that assemble the retinotectal projection and considered it an "obligation" (his words) to join this screen. One day, he called me into his office and asked me to basically drop what I was doing (my dissertation on the zebrafish olfactory system) and help him devise a suitable screening assay. Soon we had come up with a method to reliably inject two lipophilic tracer dyes into opposite poles of the larval zebrafish retina. When done by a skilled experimenter, such an injection leads to the labeling of two axon populations in green and red fluorescence, which can be followed as they navigate through the brain and terminate in two different quadrants of the tectum (Fig. 2A, B). The injection device was composed of a sharp tungsten rod inside a dye-filled cannula, which was made to vibrate by a stiff connection to a loudspeaker (see Figure 2C, D). (Later I realized that I had unknowingly re-invented the tattooing needle.) We were soon joined by Stefan Klostermann, another senior graduate student in the lab. The three of us developed methods for gentle fixation and rapid embedding of fish larvae in agarose molds (Figure 2E) and for inspection of the labeled retinotectal connections under a microscope. We also composed a tight work schedule, so that the multiple steps of the procedure could be carried out at high throughput. The logistics of transferring thousands of annotated petri dishes containing hundreds of thousands of zebrafish larvae was worked out by Freek van Eeden, Mary Mullins and others in Janni's department. Almost the entire Bonhoeffer department (see Figure 3) participated in the screen, which took about a year to complete.
In the early 1990s, a new opportunity arose in Tübingen to decipher the molecular mechanisms of retinotectal map formation in a systematic fashion. A gigantic mutagenesis screen with zebrafish was prepared in the neighboring laboratory of his colleague Christiane 'Janni' Nüsslein-Volhard (see Figure 1). Bonhoeffer recognized the power of searching for genes that assemble the retinotectal projection and considered it an "obligation" (his words) to join this screen. One day, he called me into his office and asked me to basically drop what I was doing (my dissertation on the zebrafish olfactory system) and help him devise a suitable screening assay. Soon we had come up with a method to reliably inject two lipophilic tracer dyes into opposite poles of the larval zebrafish retina. When done by a skilled experimenter, such an injection leads to the labeling of two axon populations in green and red fluorescence, which can be followed as they navigate through the brain and terminate in two different quadrants of the tectum (Fig. 2A, B). The injection device was composed of a sharp tungsten rod inside a dye-filled cannula, which was made to vibrate by a stiff connection to a loudspeaker (see Figure 2C, D). (Later I realized that I had unknowingly re-invented the tattooing needle.) We were soon joined by Stefan Klostermann, another senior graduate student in the lab. The three of us developed methods for gentle fixation and rapid embedding of fish larvae in agarose molds (Figure 2E) and for inspection of the labeled retinotectal connections under a microscope. We also composed a tight work schedule, so that the multiple steps of the procedure could be carried out at high throughput. The logistics of transferring thousands of annotated petri dishes containing hundreds of thousands of zebrafish larvae was worked out by Freek van Eeden, Mary Mullins and others in Janni's department. Almost the entire Bonhoeffer department (see Figure 3) participated in the screen, which took about a year to complete.
 This type of team effort became a model for my own future lab's modus operandi. A decade later my group at UCSF carried out a large-scale, high-throughput forward genetic screen for behavioral mutants (2005), which very much emulated the Tübingen screen. I remember a conversation I had with Friedrich Bonhoeffer, before I left Tübingen (to start a postdoc with Bill Harris). I asked him whether he thought behavioral genetics was a fruitful direction for me to follow at all. He shrugged his shoulders and only said: "Wer wagt, gewinnt", which can be loosely translated as: "No risk, no gain".
This type of team effort became a model for my own future lab's modus operandi. A decade later my group at UCSF carried out a large-scale, high-throughput forward genetic screen for behavioral mutants (2005), which very much emulated the Tübingen screen. I remember a conversation I had with Friedrich Bonhoeffer, before I left Tübingen (to start a postdoc with Bill Harris). I asked him whether he thought behavioral genetics was a fruitful direction for me to follow at all. He shrugged his shoulders and only said: "Wer wagt, gewinnt", which can be loosely translated as: "No risk, no gain".
The Tübingen mutant screen revealed numerous mutations in developmental processes that directly or indirectly steer retinal axons to their destination (1996). With his characteristic generosity, Bonhoeffer let his former postdocs pursue these mutations and later clone many of the corresponding genes in their own laboratories (notably Chi-bin Chien, Suresh Jesuthasan, Stephan Neuhauss and Rolf Karlstrom, who all joined the Bonhoeffer lab in the aftermath of the screen). The majority of the retinotectal mutants from this screen had morphological or pigmentation phenotypes and were therefore already discovered in Janni's group, before they were passed on to us retinotectal screeners. Michael Granato, Henry Roehl and Michael Brand, to name just a few, are descended from Janni's fantastic team of screeners and have contributed, in countless ways, to our current understanding of axonal pathfinding in the retinotectal system.
Friedrich Bonhoeffer was not only interested in steering axons. He was also effective in steering his surroundings. He did this by virtue of his personality, which was characterized by fairness, humility and generosity. He mentored his doctoral students and postdocs in his own unique way: by active advice as much as by omission. Both praise and criticism were applied sparingly. You had to listen carefully, and those of us who did benefitted enormously from his wisdom. His judgment could also bite. When I had just started my lab as an Assistant Professor, I went to visit him in his emeritus office in Tübingen and proudly told him about my two favorite projects. He listened attentively and then said: "The second part was quite interesting." Needless to say, after I returned home, I gave the "first part" a critical re-think.
Friedrich Bonhoeffer was born into a family – one could say: a dynasty – of scientists, doctors, theologians, musicians and scholars of all stripes. The Max Planck Institute of Biophysical Chemistry is named after his father, Karl-Friedrich. His grandfather Karl held the coveted Chair of Psychiatry and Neurology at the Charite Hospital in Berlin for 26 years. Despite an illustrious family history and his own greater-than-life achievements, Friedrich Bonhoeffer inspired all who met him with his modesty and lack of pretense, although he made no effort to hide a quiet self-confidence. Once he gave me the advice "not to read too much". He believed that other people's ideas might stifle one's own creativity.
During World War II, much of the Bonhoeffer family actively opposed Hitler and were decimated by a ruthless and vengeful regime. Among the victims of the Nazis were two of Friedrich Bonhoeffer's uncles, Dietrich (theologian and ethicist of international fame) and Klaus, as well as two of his uncles by marriage.
In November of 2020, just two months before his death, Friedrich Bonhoeffer received the prestigious Gruber Prize for Neuroscience, which he shared with Corey Goodman and Marc Tessier-Lavigne, two companions from the pioneering days of molecular developmental neurobiology. An 88 year-old Friedrich Bonhoeffer delivered a lucid presentation of his discoveries, in his characteristic no-nonsense style. Hearing him tell the story in his own words was a special reward for the listeners, many of whom had had the privilege of working with him over the decades. Friedrich Bonhoeffer risked, and he gained. He will be remembered.
Herwig Baier
(With input from Uwe Drescher and Stefan Klostermann.)




