Science Spotlight
A Multi-modal approach reveals a new role for the motile ciliary protein CFAP20 in zebrafish photoreceptors
By Uzoamaka Nwagbo
Movement and response to stimuli are some of life’s basic processes. Eukaryotes, from single-celled Paramecium to highly complex organisms like humans, regulate life processes through cilia. Cilia are specialized organelles that move to facilitate the movement of fluid (like bronchial epithelial cells) or detect sensory input (like olfactory epithelia). There are two kinds of cilia: motile cilia have an axoneme with a 9+2 microtubule organization with dynein arms that power beating movements for fluid flow and cell motility- and are associated with a heterogeneous group of human disorders characterized by infertility, chronic respiratory infections, and left-right organ reversals. On the other hand, primary (non-motile) cilia have a 9+0 microtubule organization and are involved in sensing chemicals, morphogens, and pressure, and are associated with more severe neurodevelopmental disorders. Since there was little to no overlap in diseases caused by defects in motile versus primary cilia, scientists for a long time believed in the exclusivity of primary and motile ciliopathies.
CFAP20 is a highly conserved protein that is part of the Inner Junction Hub that links the microtubule doublets in the nine-doublet outer ring. Based on previous work in unicellular flagellates, CFAP20 was thought to be required for cilium motility. Paul Chrystal, the lead author of a recent paper in Nature Communications (Chrystal et al, 2022) was thus excited to see that zebrafish cfap20 mutants had a curved body axis characteristic of a motile cilia defect. However, when the authors knocked out the C. elegans CFAP20 homolog, they were surprised to see structural defects in the microtubule doublets of non-motile sensory cilia. This finding led the authors to probe new roles for CFAP20 in other vertebrate tissues possessing primary cilia.
In zebrafish, mutants in primary cilia genes typically cause degeneration of photoreceptor cells in the retina, similar to human retinal dystrophy (Rusterholz et al., 2022). The photoreceptor outer segment is a primary cilium that is modified to form the membrane-rich lamellae containing photopigments for light detection. The authors found zebrafish cfap20 mutants have retinal degeneration, like other primary ciliopathy mutants. Thus, cfap20 has functional characteristics of both motile and primary cilium genes.
Independently, the clinicians in this study were searching for genes responsible for unexplained cases of retinal dystrophy in humans. They identified biallelic CFAP20 missense and splice-site variants in eight individuals from four unrelated families affected with retinal dystrophy. Moreover, the researchers found that the severity of the disease depends on the mutation variant inherited by the individual. Using cfap20 null mutant fish, the authors overexpressed mRNA of human missense variants of CFAP20 to test whether these human alleles were deleterious. Of the variants studied, they found that the pTyr86Cys CFAP20 variant found in humans with severe disease was unable to rescue the body axis curvature phenotype of the null zebrafish mutants. Interestingly, this allele is associated with additional phenotypes: fertility and neurological comorbidities indicative of both motile and primary cilia defects.
This study demonstrates the power of reverse genetics and a multi-species approach to understanding the etiology of protein variants in human disease. This paper has identified the inner junction as a previously undescribed part of the etiology of inherited retinal dystrophy, a known non-motile ciliopathy. The inner junction is made up of several proteins that may now define a new class of ciliopathies affecting both motile and primary cilia. However much remains to be understood about the mechanism of action of CFAP20 in cilary function. For instance, it is unclear if CFAP20 is exclusively involved in maintaining the structure of cilia or whether it is also required for its signaling capacity.
Dr. Ted Allsion described the extensive collaboration in this research project as a “leap of trust” and a consequence of “being in the right place at the right time”. Through connecting with clinicians interested in the genetic basis of the retinopathies in their patients; connecting with the Canadian Rare Diseases Models and Mechanisms (RDMM) Network; and funding from the Canadian Institutes of Health Research (CIHR), the team was able to recruit fellow experts in the field to make these groundbreaking findings. Dr. Chrystal and Dr. Allison hope these findings would lead to more research on site-directed mutagenesis of CFAP20 and other inner junction proteins to model more mutations observed in human patients, and further investigation on the mechanisms underlying organ-specific vs syndromic ciliopathies. Perhaps previously described ciliopathies should be reinvestigated for overlaps in motile and non-motile ciliary dysfunction.
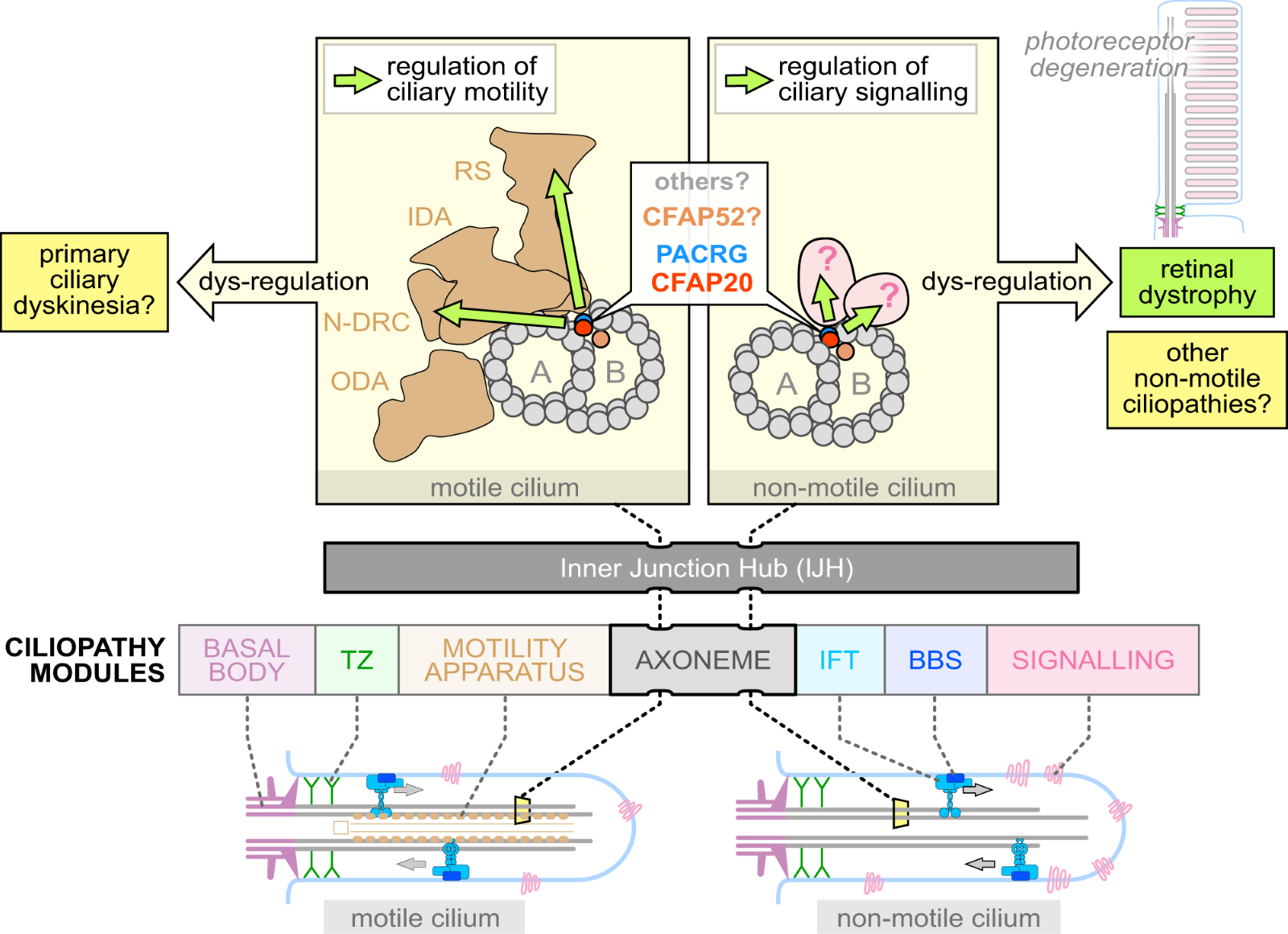
Figure 8 (From Paper by Chrystal et. al.) This diagram illustrates the Inner Junction Hub (IJH) its downstream regulatory pathways in motile (left) and non-motile (right) cilia. The major ciliopathy modules in motile cilia affect the basal body, transition zone (TZ), motility apparatus, and/or axoneme. In non-motile cilia, the main ciliopathy modules are the axoneme, intraflagellar transport (IFT) system, signaling proteins, and/or the BBS (Bardet–Biedl syndrome) trafficking system. The Bardet–Biedl syndrome trafficking system or BBSome is a complex of proteins involved in protein/cargo trafficking in the cilium. Inner Dynein Arm (IDA), Outer Dynein Arm (ODA), Radial spokes (RS), and Nexin-Dynein Regulatory Complex (N-DRC)
About the Authors
 Paul W. Chrystal and Nils J. Lambacher are equally contributing first authors of this paper. Dr. Paul W. Chrystal is now a Postdoctoral Fellow at the University of Toronto researching inherited retinal dystrophy. At the time of this publication, he was a postdoctoral Fellow in the Department of Biological Sciences, University of Alberta. He is interested in rare genetic diseases that affect the cilium, and ocular development and disease.
Paul W. Chrystal and Nils J. Lambacher are equally contributing first authors of this paper. Dr. Paul W. Chrystal is now a Postdoctoral Fellow at the University of Toronto researching inherited retinal dystrophy. At the time of this publication, he was a postdoctoral Fellow in the Department of Biological Sciences, University of Alberta. He is interested in rare genetic diseases that affect the cilium, and ocular development and disease.
 W. Ted Allison jointly supervised this work with Ping Yee Billie Au, Ian M. MacDonald, Gavin Arno, and Michel R. Leroux. He is a Professor of Biological Sciences at the University of Alberta, Edmonton, AB, Canada. He is interested in the retina, the visual system, and neurodegenerative disorders via zebrafish.
W. Ted Allison jointly supervised this work with Ping Yee Billie Au, Ian M. MacDonald, Gavin Arno, and Michel R. Leroux. He is a Professor of Biological Sciences at the University of Alberta, Edmonton, AB, Canada. He is interested in the retina, the visual system, and neurodegenerative disorders via zebrafish.
About the News Splash Science Writer
 Uzoamaka (Amaka) Nwagbo (author of this article) is a Ph.D. student in the Bernstein Lab at the University of Utah. She studies the role of very-long-chain polyunsaturated fatty acids in photoreceptor degeneration in zebrafish. When not in the lab, she enjoys spending quality time with friends and family.
Uzoamaka (Amaka) Nwagbo (author of this article) is a Ph.D. student in the Bernstein Lab at the University of Utah. She studies the role of very-long-chain polyunsaturated fatty acids in photoreceptor degeneration in zebrafish. When not in the lab, she enjoys spending quality time with friends and family.
References
Chrystal, P.W., Lambacher, N.J., Doucette, L.P. et al. (2022) The inner junction protein CFAP20 functions in motile and non-motile cilia and is critical for vision. Nat Commun 13, 6595. https://doi.org/10.1038/s41467-022-33820-w
Sources: Rusterholz, T.D.S., Hofmann C., Bachmann-Gagescu R (2022) Insights gained from zebrafish models for the ciliopathy Joubert Syndrome. Front Genet. 13:939527. doi: 10.3389/fgene.2022.939527.
Probing biomechanics in vivo - send in the robots!
By Sunandan Dhar
Many biological processes, from development to homeostasis to disease, involve physical movements: pushing, pulling, stretching, contracting, and twisting of cells and tissues. The physical properties of tissue environments vary with tissue type, age of the organism, and disease conditions such as cancer or inflammation. These environments are not static, and involve dynamic forces generated by cells. The interplay of such forces plays a key role in collective migration and patterning of groups of cells during morphogenesis or wound repair.
Although a large number of biochemical and molecular biology tools have been developed to understand processes within our bodies, very few tools exist to study biomechanics in vivo. Existing techniques to precisely measure the mechanical properties of tissues, such as atomic force microscopy, cannot be performed on internal tissues in vivo [Gautier et al., 2015]. Live imaging-based techniques such as elastography and Brillouin microscopy are promising for in vivo applications, but have low spatial resolution at the cellular-scale [Garra, 2015; Scarcelli & Yun, 2008]. Advances in traction force microscopy have enabled the measurement of active forces in in vitro models [Style et al., 2014]. However, it remains a challenge to measure these forces in vivo without perturbing natural processes.
Mohagheghian et al. now report an innovative solution to tackle this by developing a custom ‘microrobot’ to probe multiple mechanical properties in vivo. Co-first author Junyu Luo explains: “We usually use separate probes to measure stiffness and traction forces at different sites; the motivation was to be able to measure both at the same time and place”. The device consists of a poly(ethylene glycol) gel that encapsulates a magnetic cross-shaped apparatus and fluorescent particles. It can be implanted in vivo to sequentially quantify stiffness and traction forces of a tissue.
As a proof-of-concept, the authors injected the microrobots into zebrafish blastulas (4 hpf) to measure mechanical properties inside developing embryos. According to Junyu, “the large size of zebrafish embryos made it much easier to inject and test the probe than mouse embryos”. To measure tissue stiffness inside the embryo, the magnetic microcross was rotated by applying an external magnetic field. Stiff tissues are expected to resist the rotation more than softer ones. Thus, the degree of rotation, observed under a microscope, provides a quantitative readout of tissue stiffness. Embryos were found to have an average stiffness of 242 Pa, similar to that of a soft gel, but varied based on the location of the probe. It is known that zebrafish embryos are viscoelastic [Betz, 2021], and the authors are able to resolve the viscous and elastic components of stiffness based on the timing of rotations.
After characterising the stiffness, the authors then measured traction forces using the fluorescent nanoparticles dispersed within the gel. The gel is initially rigid but can be softened significantly using short pulses of ultraviolet light. The softened gel is then freely deformed by any forces active in the local environment, causing the nanoparticles within it to move around (Figure A-iv). Another co-first author, Dr Fuxiang Wei describes, “measuring the displacement of the fluorescent particles allows us to calculate the amount and directions of tractions forces at the site”.
In the zebrafish embryo, the authors found that traction forces were significantly larger inside the embryo than at the embryo-yolk boundary. This presents an opportunity to study how cell-generated forces change over time, for instance, during epiboly movements. Previous studies using microdroplets or beads embedded within the embryo have reported similar normal (perpendicular) traction forces [Campas et al., 2014; Traber et al., 2019], but the probe design in this study also allowed for measurement of previously unreported shear forces.
The authors used the microrobot to perform similar measurements on cancer cell colonies grown in vitro and in live mouse embryos. Dr Wei mentions that the team is “trying to build smaller devices to make it easier to inject into embryos without damaging the tissue”. However, the current design only allows a one-time measurement since stiffness cannot be remeasured once the gel is softened by UV light. Using gels with reversible softening and stiffening may enable sequential measurements at the site over time as the organism develops. A key consideration is that this technique is limited by the penetrance of the external magnetic field and UV light to the site of interest, and the ability to perform live imaging to make measurements. In adults, this will likely only be possible in superficial tissues. Further innovations are needed to probe mechanical properties during aging and disease in adult fish. Nonetheless, this approach is a crucial addition to our toolkit in the quest to unravel the physics of life.
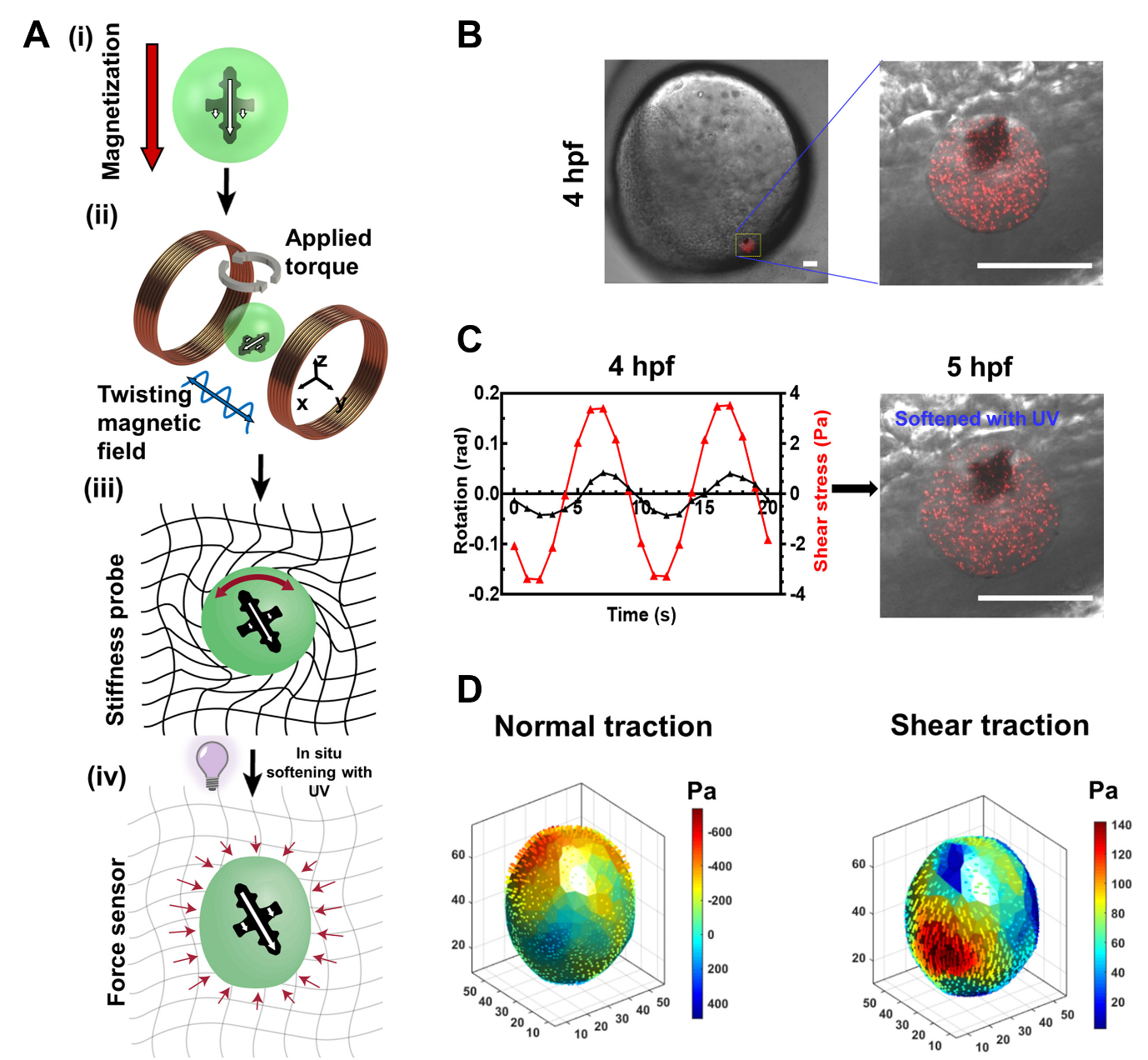
Figure adapted from Mohagheghian et al. (2023). A: Schematic showing the operation of the microrobot. B: The probe, containing fluorescent nanoparticles (red dots), is injected into a zebrafish blastula at 4 hpf (scale bar = 50 µm). C: A sinusoidal magnetic field is applied (red line) and the resulting probe rotation is measured (black line). After this measurement, the gel is softened by UV light. D: Maps of normal and shear traction forces are generated based on the movements of the fluorescent nanoparticles in the gel.
References:
-
Betz, T. (2021). Viscoelastic properties driving collective migration in zebrafish development. In Viscoelasticity and Collective Cell Migration (pp. 135-155). Academic Press.
-
Campàs, O., Mammoto, T., Hasso, S., Sperling, R.A., O'connell, D., Bischof, A.G., Maas, R., Weitz, D.A., Mahadevan, L. and Ingber, D.E. (2014). Quantifying cell-generated mechanical forces within living embryonic tissues. Nature methods, 11(2), pp.183-189.
-
Garra, B. S. (2015). Elastography: history, principles, and technique comparison. Abdominal imaging, 40, 680-697.
-
Gautier, H. O., Thompson, A. J., Achouri, S., Koser, D. E., Holtzmann, K., Moeendarbary, E., & Franze, K. (2015). Atomic force microscopy-based force measurements on animal cells and tissues. In Methods in cell biology (Vol. 125, pp. 211-235). Academic Press.
-
Mohagheghian, E., Luo, J., Yavitt, F.M., Wei, F., Bhala, P., Amar, K., Rashid, F., Wang, Y., Liu, X., Ji, C. and Chen, J. (2023). Quantifying stiffness and forces of tumor colonies and embryos using a magnetic microrobot. Science Robotics, 8(74), p.eadc9800.
-
Scarcelli, G., & Yun, S. H. (2008). Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nature photonics, 2(1), 39-43.
-
Style, R.W., Boltyanskiy, R., German, G.K., Hyland, C., MacMinn, C.W., Mertz, A.F., Wilen, L.A., Xu, Y. and Dufresne, E.R. (2014). Traction force microscopy in physics and biology. Soft matter, 10(23), pp.4047-4055.
-
Traeber, N., Uhlmann, K., Girardo, S., Kesavan, G., Wagner, K., Friedrichs, J., Goswami, R., Bai, K., Brand, M., Werner, C. and Balzani, D. (2019). Polyacrylamide bead sensors for in vivo quantification of cell-scale stress in zebrafish development. Scientific reports, 9(1), p.17031.
About the Authors
 Junyu Luo is a graduate student at College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China. She is studying cellular forces and their role in embryonic development under the guidance of Dr. Ning Wang.
Junyu Luo is a graduate student at College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China. She is studying cellular forces and their role in embryonic development under the guidance of Dr. Ning Wang.
 Dr. Fuxiang Wei is an Associate Professor at College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China. Dr. Wei focusses on stress-induced gene expression in living systems, tissue stiffness, and traction force measurements. 
Dr. Fuxiang Wei is an Associate Professor at College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China. Dr. Wei focusses on stress-induced gene expression in living systems, tissue stiffness, and traction force measurements. 
 Dr. Ning Wang is currently the Hoeft Professor in Engineering at University of Illinois at Urbana-Champaign (UIUC) and leads the mechanobiology lab there. Dr. Wang has worked on mechanotransduction and mechanobiology for decades after receiving ScD in Physiology and postdoctoral training at Harvard in early 90s. He became a faculty member at Harvard School of Public Health in 1994 and joined the faculty of UIUC in 2006. He will join the faculty of Bioengineering at Northeastern University in Boston in July 2023. Dr. Wang is interested in roles of forces and mechanics in development and physiology and diseases such as cancer.
Dr. Ning Wang is currently the Hoeft Professor in Engineering at University of Illinois at Urbana-Champaign (UIUC) and leads the mechanobiology lab there. Dr. Wang has worked on mechanotransduction and mechanobiology for decades after receiving ScD in Physiology and postdoctoral training at Harvard in early 90s. He became a faculty member at Harvard School of Public Health in 1994 and joined the faculty of UIUC in 2006. He will join the faculty of Bioengineering at Northeastern University in Boston in July 2023. Dr. Wang is interested in roles of forces and mechanics in development and physiology and diseases such as cancer.

Sunandan Dhar is a graduate student at the Mechanobiology Institute, National University of Singapore. Under the supervision of Dr Timothy Saunders, his research focusses on the interplay of signalling and mechanics driving muscle fibre morphogenesis in a zebrafish model. He enjoys learning about the latest advances across disciples that aid in our understanding of biology.




